Question 1.
State Mendeleev s Periodic law.
Answer:
Medeleev’s periodic law states that the properties of elements are periodic function of their atomic masses.
Question 2.
Why did Mendeleev leave some gaps in the Periodic table?
Answer:
Mendeleev left some gaps in the periodic table for yet to be discovered elements. Mendeleev predicted the properties of these elements on the basic of their positions. For example, he predicted the properties of gallium (eka-aluminium) and germanium (eka-silicon) which were unknown at that time.
Question 3.
If the letter ‘R’ was used to represent any of the elements in the group, then the hydride and oxide of carbon would respectively be represented as
(a) RH4, RO
(b) PH4, RO2
(c) RH2, RO2
(d) RH2, RO
Answer:
(b) CH4 is written for hydride and CO2 is written for oxide of carbon.
Question 4.
Isotopes are
(a) atoms of an element with similar chemical properties but different atomic masses
(b) atoms of different elements with similar chemical properties but different atomic masses
(c) atoms of an element with different chemical properties but same atomic masses
(d) atoms of different elements with different chemical properties but same atomic masses. (2020)
Answer:
(a) Isotopes have same atomic number, hence similar chemical properties and different atomic masses.
Question 5.
How many metals are present in second period of periodic table? (2020)
Answer:
Two metals (lithium and beryllium) are present in second period of periodic table.
Question 6.
On the basis of electronic configuration of 95X, the group number and period of the element ‘X’ is
(a) group 15 period 2
(b) group 13 period 2
(c) group 9 period 5
(d) group 13 period 5. (2020)
Answer:
(b) For element X of atomic number 5, the electronic configuration is 2,3. So, it has 3 valence electrons and hence it belongs to group 13. As five electrons are filled in two shells, so it belongs to 2nd period. Question 7.
An element ‘X’ with atomic number 11 forms a compound with element ‘Y’ with atomic number 8. The formula of the compound formed is
(a) XY
(b) X2Y
(c) XY2
(d) X2Y3 (2020)
Answer:
(b): The electronic configuration of X is 2, 8, 1. Hence, it belongs to group 1. Its valency is 1.
The electronic configuration of Y is 2, 6. Hence, it belongs to group 16. Its valency is 2
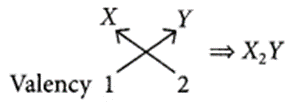
Question 8.
Define electropositivity. (2020)
Answer:
Electropositivity is the measure of the ability of elements (mainly metals) to donate electrons to form positive ions.
Question 9.
The atomic radii of first group elements are given below:
| Group-I element | Atomic Radii (pm) |
| Na | 86 |
| K | 231 |
| Rb | 244 |
| Cs | 282 |
State the reason behind the observed trend in the above elements. (2020)
Answer:
On moving down the group, the new shells are being added. This increase the distance between outermost shell and nucleus, hence the atomic radii of elements increase gradually down the group in spite of the increase in nuclear change.
Question 10.
An element ‘X’ is forming an acidic oxide. Its position in modern periodic table will be
(a) group 1 and period 3
(b) group 2 and period 3
(c) group 13 and period 3
(d) group 16 and period 3. (2020)
Answer:
(d) : As the element X forms an acidic oxide, hence ‘X is a non-metal. Hence, X is sulphur.
Question 11.
Consider the following statements about an element ‘X with number of protons 13.
(A) It forms amphoteric oxide.
(B) Its valency is three.
(C) The formula of its chloride is XCl3.
The correct statements is/are
(a) only (A)
(b) only (B)
(c) (A) and (C)
(d) (A), (B) and (C). (2020)
Answer:
(d) The number of protons in X is 13. Hence, its atomic number is 13. The electronic configuration of X is 2, 8, 3. Hence, it is a group 13 element i.e., X is aluminium. Aluminium forms amphoteric oxide, its valency is 3 and formula of aluminium chloride is AlCl3.
Question 12.
Write the number of valence electrons present in a nitrogen atom (147N). (2020)
Answer:
The atomic number of nitrogen is 7. Its electronic configuration is 2, 5. The number of valence electrons in it is five.
Question 13.
Write the number of vertical columns in the Modern Periodic Table. What are these columns called? (Delhi 2014, 2013)
Answer:
There are 18 vertical columns in the Modern periodic table which are called groups.
Question 14.
Write the number of horizontal rows in the Modern Periodic Table. What are these rows called? (Delhi 2014)
Answer:
There are seven horizontal rows of elements in the Modern periodic table which are known as periods.
Question 15.
Write any one difference in the electronic configurations of group 1 and group 2 elements. (Delhi 2014)
Answer:
Group 1 elements have one electron in their outermost shell while group 2 elements have two electrons in their outermost shell.
Question 16.
List any two properties of the elements belonging to the first group of the Modern Periodic Tablet (AI 2014)
Answer:
Two properties of the elements belonging to the first group:
(i) As the elements belong to group 1, so they have one electron in their outermost shell hence, valency of these elements is one.
(ii) Alkali metals (group 1 elements) are electropositive in nature.
Question 17.
Write the atomic numbers of two elements ‘X’ and ‘Y’ having electronic configurations 2, 8, 2 and 2, 8, 6 respectively. (AI 2014)
Answer:
Electronic configuration of X = 2, 8, 2
∴ Atomic number = 2+ 8 + 2 = 12 Similarly,
Electronic configuration of Y = 2, 8, 6
∴ Atomic number = 2 + 8 + 6 = 16
Question 18.
The atomic numbers of three elements A, B and C are 12, 18 and 20 respectively. State giving reason, which two elements will show similar properties. (AI 2014)
Answer:
Atomic number of A = 12
∴ Electronic configuration = 2, 8, 2
Similarly, for B(18) = 2, 8, 8
for C(20) = 2, 8, 8, 2
As elements A and C contain two valence electrons in their outermost shell (group-2) they will show similar properties.
Question 19.
State the Modern periodic law of classification of elements. (Foreign 2014)
Answer:
Modern periodic law states that the physical and chemical properties of elements are the periodic function of their atomic numbers.
Question 20.
Out of the three elements P, Q and R having atomic numbers 11, 17 and 19 respectively, which two elements will show similar properties and why? (Foreign 2014)
Answer:
Atomic number of P = 11 Electronic configuration of P = 2, 8,1 Electronic configuration of Q(17) = 2, 8, 7 and for R(19) = 2, 8, 8, 1
Thus, from electronic configurations of P and R, it is observed that they belong to group 1 as both have one valence electron and have valency equal to 1. Thus, P and R will have similar properties.
Question 21.
Write the formula used to determine the maximum number of electrons which a shell in an atom can accommodate. (Foreign 2014)
Answer:
The maximum number of electrons that can be accommodated in a shell, is given by the formula 2n², where ‘n is the number of the shell.
Question 22.
How it can be proved that the basic structure of the Modern Periodic Table is based on the electronic configuration of atoms of different elements? (Delhi 2019)
Answer:
Electronic configuration of an element decides its position in Modern periodic table.
Lets take an example of sodium (Na).
Atomic number of sodium = 11
Thus, electronic configuration of Na = 2, 8, 1 As Na contains 1 electron in its outermost shell, it belongs to group 1. Sodium contains 3 shells so, it belongs to period number 3.
Thus, we can conclude that
Group number = Number of valence electrons
(When valence electrons are 1 and 2) and group number = 10 + valence electrons
(When valence electrons are 3 and above) Period number = Number of shells in which electrons are filled.
Question 23.
The electronic configuration of an element is 2, 8, 4. State its
(a) group and period in the Modern Periodic Table.
(b) name and write its one physical property. (Delhi 2019)
Answer:
(a) The element belongs to group 14 and 3rd period of the Modern Periodic Table.
(b) The element is silicon. It is non-lustrous. Question 24.
An element X has atomic number 13 :
(a) Write its electronic configuration.
(b) State the group to which ‘X’ belongs?
(c) Is ‘X a metal or a non-metal?
(d) Write the formula of its bromide. (Delhi 2012)
Answer:
X has atomic number = 13
(a) Electronic configuration of X = 2, 8, 3
(b) As X contains 3 valence electrons in its outermost shell, it belongs to group 13.
(c) X is a metal as it contains 3 valence electrons which can be lost easily.
(d) Formula of X with bromine will be
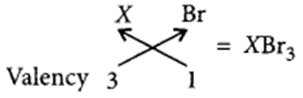
Question 25.
How can the valency of an element be determined if its electronic configuration is known? What will be the valency of an element of atomic number 9(nine)? (Delhi 2012, 2011)
Answer:
Valency of an element is determined by the number of electrons present in its outermost shell. For elements having outermost electrons 1 to 4, valencies are equivalent to their respective valence electrons.
For elements having outermost electrons 5 to 8, valency is calculated as;
Valency = 8 – (Number of valence electrons)
For element having atomic number = 9
Electronic configuration = 2, 7
Valency = 8 – 7 = 1
Question 26.
Choose from the following :
6C, 8O, 10Ne, 11Na, 14Si
(i) Elements that should be in the same period.
(ii) Elements that should be in the same group.
State reason for your selection in each case. (AI 2012)
Answer:
The electronic configurations of the given elements are:
6C = 2, 4
8O = 2, 6
10Ne = 2, 8
11Na = 2, 8,1
14Si = 2, 8, 4
(i) 6C, 8O, 10Ne, all contain two shells hence, they belong to same period i.e., second period.
11Na, 14Si both contain three shells hence, they belong to third period.
(6C, 8O, 10Ne) ⇒ period number 2
(11Na, 14Si) ⇒ period number 3
(ii) 6C and 14Si belong to the same group as they both contain 4 electrons in their outermost shell. Thus, 6C and 14Si belong to group 14.
Question 27.
An element ‘X’ belongs to 3rd period and group 17 of the periodic table. State its
(i) electronic configuration, (ii) valency Justify your answer with reason. (AI 2012)
Answer:
As element X belongs to group 17, it will have 7 electrons in its outermost shell. Moreover, X belongs to period number 3 so, it will have 3 shells.
(i) Electronic configuration of X = 2, 8, 7
(ii) Valency of element X
= 8 – (Number of valence electrons) = 8 – 7 = 1
Question 28.
Choose from the following :
4Be, 9F, 19K, 20Ca
(i) The element having one electron in the outermost shell.
(ii) Two elements of the same group. (Foreign 2012)
Answer:
The electronic configurations of the given elements are:
4Be = 2, 2
9F = 2, 7
19K = 2, 8, 8,1
20Ca = 2, 8, 8, 2
(i) Potassium (K) has one electron in its outermost shell.
(ii) Be and Ca have two electrons in their outermost shells hence, they belong to same group.
Question 29.
An element has atomic number 13.
(a) What is the group and period number to which this element belongs?
(b) Is this element a metal or a non-metal? Justify your answer. (Foreign 2012)
Answer:
Atomic number of element = 13
Thus, its electronic configuration = 2, 8, 3
(a) From the electronic configuration, it can be easily seen that there are 3 electrons in the outermost shell which indicates that it belongs to group number 10 + 3 = 13.
Moreover, the element has 3 shells in which electrons are filled thus, it belongs to period number 3.
(b) As the element contains 3 valence electrons which can be easily lost thus, it is a metal.
Question 30.
How does the electronic configuration of an
atom of an element relate to its position in the Modern Periodic Table? Explain with one example. (Delhi 2011)
Answer:
Refer to answer 22.
Question 31.
How does the valency of elements vary (i) in going down a group, and (ii) in going from left to right in a period of the periodic table? (AI 2011)
Answer:
(i) When we go down the group the valency of elements remains same.
(ii) When we move along the period from left to right, the valency of elements first increases and then decreases.
Question 32.
In the Modern Periodic Table, the element calcium (atomic number = 20) is surrounded by elements with atomic numbers 12, 19, 21 and 38. Which of these elements has physical and chemical properties resembling those of calcium and why? (AI 2011)
Answer:
From the given data, the electronic configuration of different elements can be written as:
Calcium (20) = 2, 8, 8, 2
Element with atomic number 12 = 2, 8, 2
Element with atomic number 19 = 2, 8, 8,1
Element with atomic number 21 = 2, 8, 8, 3
Element with atomic number 38 = 2, 8,18, 8, 2
It can be easily seen that elements with atomic numbers 12 and 38 have two electrons in their outermost shell thus, they belong to same group as that of calcium. So, they will show the physical and chemical properties resembling those of calcium.
Question 33.
In the periodic table, how does the tendency of atoms to loose electrons change on going from
(i) left to right across a period?
(ii) top to bottom in a group? (Foreign 2011)
Answer:
(i) Tendency of atoms to loose electrons decreases from left to right in a period due to increase in effective nuclear charge.
(ii) Tendency of atoms to loose electrons increases down the group due to increase in atomic radii.
Question 34.
What is meant by periodicity of properties of elements? Why are the properties of elements placed in the same group of the periodic table similar? (Foreign 2011)
Answer:
When elements are arranged in increasing order of their atomic numbers, elements with similar chemical properties are repeated at definite intervals. Ibis is known as periodicity of properties of elements.
Elements placed in the same group of the periodic table have similar properties because they have same number of outermost electrons and hence, show same valency. Thus, they all will form similar type of compounds
Question 35.
The position of three elements A, B and C in the modern periodic table is as follows :
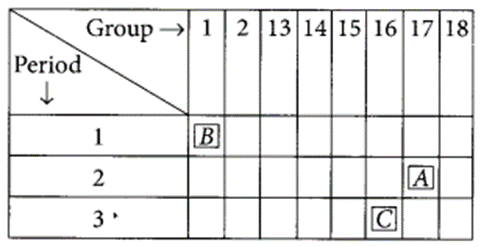
(a) Write formula of compound formed between:
(i) B and A (ii) B and C
(b) Is any of the three elements a metal? Give reason to justify your answer. (2020)
Answer:
(a) (i) B belongs to group 1, hence its valency is 1.
A belongs to group 17, hence its valency is also 1.
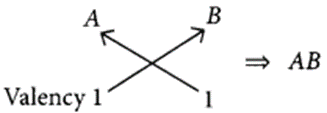
(ii) C belongs to group 16, hence, its valency is 2.
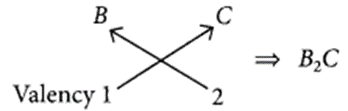
(b) As B belongs to group 1, it contains one valence electron which can be easily lost. Hence, B is a metal.
Question 36.
Three elements X, Y and Z have atomic numbers 7, 8 and 9 respectively.
(a) Arrange them in the decreasing order of their atomic radii.
(b) Which of the three is most electronegative? Why?
(c) Write the formula of compound formed between
(i) X and Y (ii) X and Z (2020)
Answer:
For element X of atomic number 7, the electronic configuration is 2, 5 so it has 5 valence electrons and hence, it belongs to group 15. As seven electrons are filled in two shells so, it belongs to 2nd period.
Similarly, for 7(8), electronic configuration = 2, 6
Period number = 2, Group number = 16 and for Z(9) = 2, 7
Period number = 2, Group number = 17 (a) As size of the atoms decreases on moving from left to right in a period so, the order of atomic radii will be : X > Y > Z.
(b) As electronegativity increases in moving left to right in a period so, the most electronegative element will be Z.
(c) (i) Formula of the compound when X combines with Y
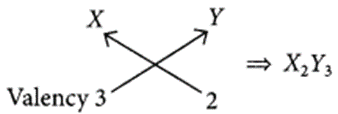
(ii) Formula of the compound when X combines with Z:
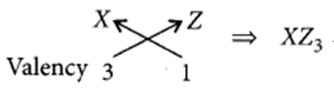
Question 37.
Based on the group valency of elements write the molecular formula of the following compounds giving justification for each:
(i) Oxide of first group elements
(ii) Halide of the elements of group thirteen, and
(iii) Compound formed when an element, A of group 2 combines with an element, B of group seventeen. (Delhi 2019)
Answer:
(i) Oxides of group 1 elements :
Let the element be A.
As A belongs to group 1 of the periodic table, it will have valency = 1.
So, chemical formula of its oxide will be A O
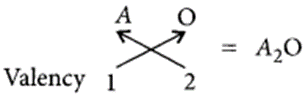
(ii) Halides of the element of group-13 :
Let the element be D.
As D belongs to group 13, it will have valency = 3 Halide X has the valency = 1
So, chemical formula will be
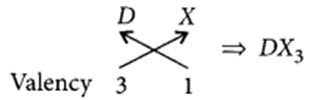
(iii) Valency of A = 2
Valency of B = 1
Chemical formula of the compound will be
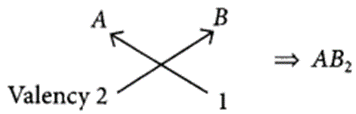
Question 38.
Write the names given to the vertical columns and horizontal rows in the Modern Periodic Table. How does the metallic character of elements vary on moving down a vertical column? How does the size of atomic radius vary on moving left to right in a horizontal row? Give reason in support of your answer in the above two cases. (Delhi 2017)
Answer:
In Modern periodic table, there are 18 vertical columns called groups and 7 horizontal rows called periods.
The elements which have a greater tendency to loose electrons are more metallic thus, the metallic character of elements increases down the group as their tendency to loose electrons increases. Atomic radius decreases as we move from left to right in a horizontal row. At each successive element, the electron enters to the same shell due to which there is increase in nuclear charge and the electrons are pulled with greater attractive force. Hence, the atomic size decreases.
Question 39.
An element P (atomic number 20) reacts with an element Q (atomic number 17) to form a compound. Answer the following question giving reason:
Write the position of P and Q in the Modern Periodic Table and the molecular formula of the compound formed when P reacts with Q. (Delhi 2017)
Answer:
Atomic number of P = 20
∴ Electronic configuration of P = 2, 8, 8, 2
Atomic number of Q = 17
∴ Electronic configuration of Q = 2, 8, 7 As P contains 4 shells, it belongs to 4th period and due to presence of two valence electrons, it belongs to 2nd group.
Similarly, Q contains 3 shells and 7 valence electrons thus, it belongs to 3rd period and 17th (10 + 7) group.
The molecular formula of compound formed when P reacts with Q will be :
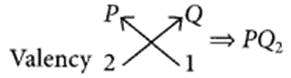
Question 40.
Write the number of periods and groups in the Modern Periodic Table. How does the metallic character of elements vary on moving (i) from left to right in a period, and
(ii) down a group? Give reason to justify your answer. (AI 2017)
Answer:
In the Modern periodic table, there are 18 vertical columns called groups and 7 horizontal rows called periods.
Trend of metallic character :
(i) Along the period from left to right: Metallic character of elements decreases as we move from left to right in a period. Metallic character depends on the electropositive character (tendency to loose electrons) of the elements. As we go across the period from left to right, one electron is added to same shell at every stage which increases the effective nuclear charge and hence, valence electrons becomes more and more closer to the nucleus. Due to this, the tendency of atoms to loose valence electrons and form positive ions decreases. Hence, electropositive character decreases resulting in decrease of metallic character.
(ii) Down the group : Metallic character of elements increases on moving down the group as the electropositive character increases down the group.
Question 41.
Na, Mg and Al are the elements of the 3rd periods of the Modern Periodic Table having group number 1,2 and 13 respectively. Which one of these elements has the
(a) highest valency, (b) largest atomic radius, and (c) maximum chemical reactivity? Justify your answer stating the reason for each. (AI 2017)
Answer:
Period number of Na, Mg and Al = 3
Group numbers of Na, Mg and Al are 1, 2 and 13 respectively.
(a) Aluminium (Al) will show highest valency of +3 as it belongs to group number 13 (valency = 13 – 10 = 3). Moreover, along the period from left to right valency first increases to maximum (+4) and then decreases.
(b) Sodium (Na) will have the largest atomic radius because as we move along the period from left to right, the atomic radius decreases.
(c) Sodium (Na) will have maximum chemical reactivity because as we move along the period from left to right, chemical reactivity of metals decreases.
Question 42.
Calcium is an element with atomic number 20. Stating reason answer each of the following questions:
(i) Is calcium a metal or non-metal?
(ii) Will its atomic radius be larger or smaller than that of potassium with atomic number 19?
(iii) Write the formula of its oxide. (Delhi 2016)
Answer:
Given that, atomic number of calcium is 20.
So, its electronic configuration = 2, 8, 8, 2 .
(i) As, it has 2 valence electrons in the outermost shell which can be easily lost, so it is a metal.
(ii) Atomic number of K (potassium) is 19 so, it is placed before Ca(20) in the same period.
On moving from left to right in a period, the atomic radius decreases.
Hence, atomic radius of Ca(20) will be smaller than that of K(19).
(iii) The valency of calcium as well as oxygen is 2 thus, the formula of the oxide will be CaO.
Question 43.
An element M with electronic configuration (2, 8, 2) combines separately with (NO−3), (SO2−4) and (PO3−4) radicals. Write the formula of the three compounds so formed. To which group and period of the Modern Periodic Table does the element M belong? Will M form covalent or ionic compounds? Give reason to justify your answer. (Delhi 2016)
Answer:
Electronic configuration of M is 2, 8, 2 which shows that it belongs to group 2 and period 3 of the Modern periodic table.
As it has 2 valence electrons, so the valency of element M will be 2.
The chemical formulae of the compounds formed will be
M(NO3)2,MSO4, M3(PO4)2
As M has two valence electrons, it can easily loose these electrons to attain a noble gas configuration. Hence, M will form ionic compounds.
Question 44.
Name any two elements of group one and write their electronic configurations. What similarity do you observe in their electronic configurations? Write the formula of oxide of any of the aforesaid element. (Delhi 2016)
Answer:
Two elements of group 1 are sodium (Na) and potassium (K).
Electronic configuration of Na (11) = 2, 8, 1
Electronic configuration of K (19) = 2, 8, 8, 1
From the electronic configuration, we observe that both (Na and K) have one electron in outermost shell due to which they have valency equal to one.
Thus, formula of their oxides are, Na2O and K2O.
Question 45.
Two elements A and B belong to the 3rd period of Modern Periodic Table and are in group 2 and 13 respectively. Compare their following characteristics in tabular form.
(a) Number of electrons in their atoms
(b) Size of their atoms
(c) Their tendencies to loose electrons
(d) The formula of their oxides
(e) Their metallic characters
(f) The formula of their chlorides (Delhi 2016)
Answer:
Electronic configuration of A = 2, 8, 2 i.e., Mg
Electronic configuration of B = 2, 8, 3 i.e., Al
| Characteristics | A | B |
| (a) No. of electrons in their atoms | 12 | 13 |
| (b) Size of their atoms | Bigger | Smaller |
| (c) Tendency to loose electrons | More | Less |
| (d) Formula of their oxides | AO | B2O3 |
| (e) Metallic character | More | Less |
| (f) Formula of their chlorides | ACl2 | BCl2 |